Facility
The BEACON manufacturing facility is committed to upholding the highest standards of quality, safety, and compliance throughout our operations. The company adheres to Good Manufacturing Practice (GMP) standards, focusing on contamination prevention, proper flow design, and containment systems. Manufacturing processes follow strict GMP guidelines, and production machines are equipped with containment systems while following CFR Part 11 requirements for data auditing.
We comply with Good Engineering Practices (GEP) for utilities and maintain Good Laboratory Practices (GLP) in laboratory operations. All systems and processes undergo rigorous validation and qualification procedures. Our sterile manufacturing processes fully comply with EU standards, and we prioritize personnel hygiene and safety through proper gowning and hygiene protocols. Environmental, health, and safety are the prime, with adherence to ISO standards. The warehouse operations meet international GMP standards for the storage of materials and finished goods.

Oral & Solid Facilities
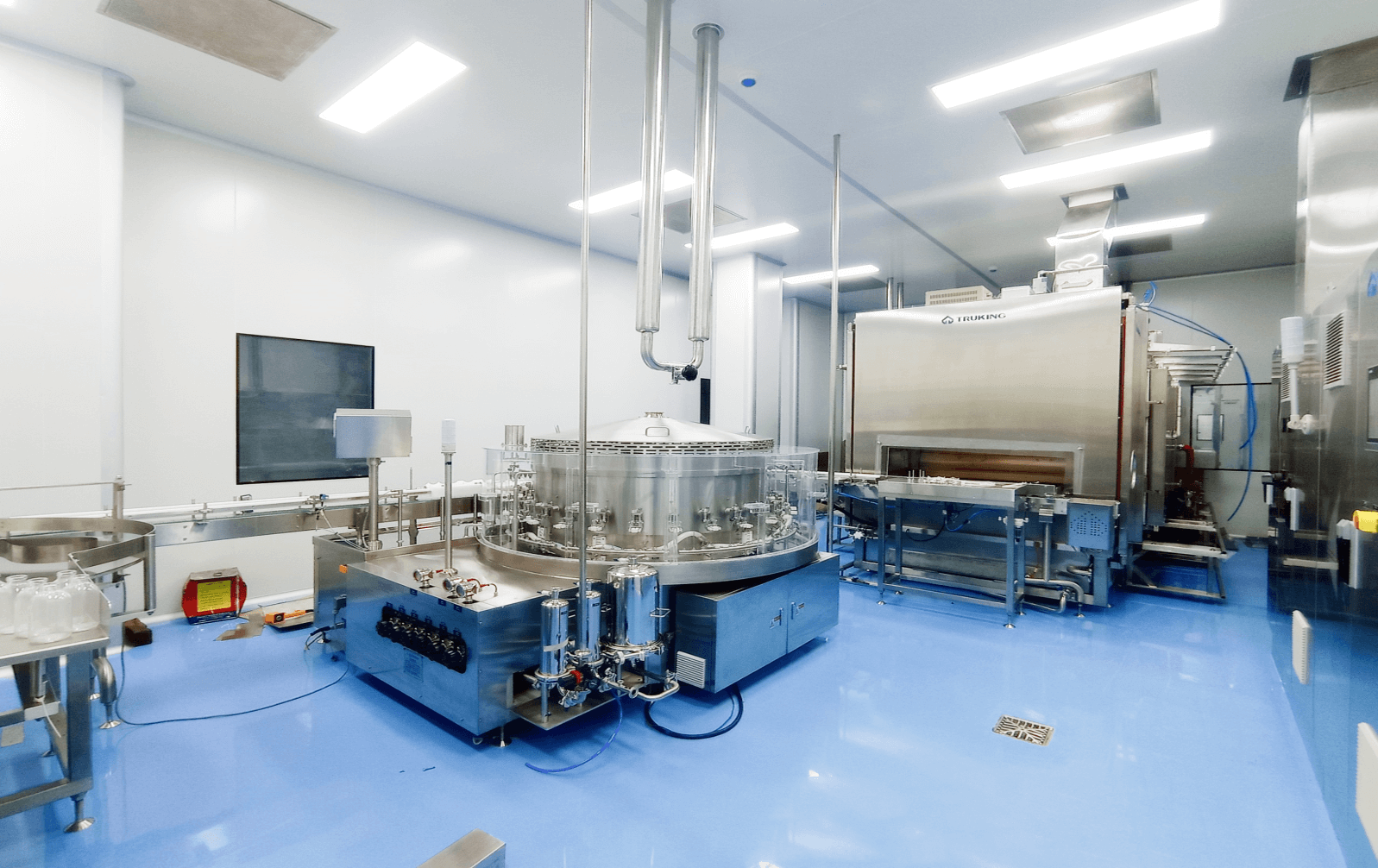
Parenteral Production Facilities

Oncology Facilities