What is Tirzix?
Tirzix contains Tirzepatide that is used to help lose weight in patients with obesity or overweight and reduce blood sugar in patient with type-2 diabetes. It is a once-weekly subcutaneous injectable for adults, engineered from the naturally occuring GIP sequence, with agonist activity at both the GIP(Gastric Inhibitory Polypeptide) and GLP-1(Glucacon-Like Peptide 1) receptors.
• Tirzepatide(Tirzix) is a pre-filled injectable prescription medicine that may help adults with obesity, or with excess weight (overweight) who also have weight-related medical problems, lose weight.
• Tirzepatide(Tirzix) should be used with a reduced-calorie diet and increased physical activity.
• Tirzepatide(Tirzix) should not be used with other Tirzepatide-containing products or any GLP-1 receptor agonist medicines.
• It is not known if Tirzepatide(Tirzix) is safe and effective when taken with other prescription, over-the-counter, or herbal weight loss products.
• It is not known if Tirzepatide(Tirzix) can be used in people who have had pancreatitis.
• It is not known if Tirzepatide(Tirzix) is safe and effective for use in children under 18 years of age.
• The BMI is defined as the body mass divided by the square of the body height, and is expressed in units of kg/m2, resulting from mass in kilograms (kg) and height in metres (m).


Mechanism of Action:
- 1
Tirzepatide may reduce blood sugar from the 1st dose.
- 2
Tirzepatide helps to release insulin when the blood sugar level is high.
- 3
Tirzepatide helps to remove excess sugar from the blood.
- 4
Tirzepatide stops the liver from making excess sugar and releases sugar into the blood.
- 5
Tirzepatide reduces food intake and slows down the process of food emptying.
Weight Loss Statistics
Tirzepatide ensures a profound reduction in Body Weight for Diabetic and Non-Diabetic Patients
When paired with diet and exercise, Tirzepatide can both lower blood glucose(sugar) levels and lead to significant weight loss in people with diabetes. And it has been shown to cause weight loss in people without diabetes, too.
| Category | Dose | Weight Reduction (lbs) | Avg Weight Reduction (%) | Avg Starting Weight (lbs) |
|---|---|---|---|---|
| Non-Diabetic | Tirzepatide 10 mg |
44 | 19.5 | 233.3 |
| Diabetic | Tirzepatide 10 mg |
28 | 12.8 | 222.4 |
| Non-Diabetic | Tirzepatide 15 mg |
48 | 20.9 | 232.8 |
| Diabetes | Tirzepatide 15 mg |
33 | 14.7 | 224.2 |
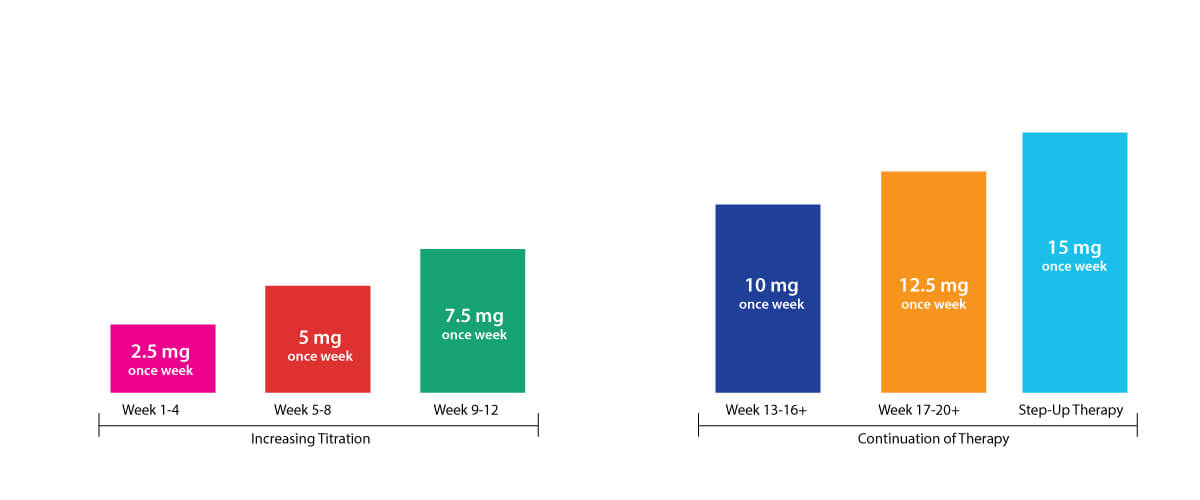
Dosing Schedule
The recommended starting and maximum dosage of Tirzepatide is 2.5 mg and 15 mg subcutaneous injection. Each pre-filled injection is administered once in a week. The dosage schedule is illustrated below-
Medication Guideline
What is the most important information I should know about Tirzepatide?
Tirzepatide may cause serious side effects, including:
• Possible thyroid tumors, including cancer. Tell your healthcare provider if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyroid cancer. In studies with rats, Tirzepatide and medicines that work like Tirzepatide caused thyroid tumors, including thyroid cancer. It is not known if Tirzepatide will cause thyroid tumors, or a type of thyroid cancer called medullary thyroid carcinoma (MTC) in people.
• Do not use Tirzepatide if you or any of your family have ever had a type of thyroid cancer called MTC, or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
Do not use Tirzepatide if:
• you or any of your family have ever had a type of thyroid cancer called MTC or if you have an endocrine system condition called MEN 2.
• you have had a serious allergic reaction to Tirzepatide or any of the ingredients in Tirzepatide.Before using Tirzepatide, tell your healthcare provider about all of your medical conditions, including if you:
• have or have had problems with your pancreas or kidneys.
• have severe problems with your stomach, such as slowed emptying of your stomach (gastroparesis) or problems with digesting food.
• have a history of diabetic retinopathy.
• are pregnant or plan to become pregnant? Tirzepatide may harm your unborn baby. Tell your healthcare provider if you become pregnant while using Tirzepatide.
• Birth control pills by mouth may not work as well while using Tirzepatide. If you take birth control pills by mouth, your healthcare provider may recommend another type of birth control for 4 weeks after you start Tirzepatide and for 4 weeks after each increase in your dose of Tirzepatide. Talk to your healthcare provider about birth control methods that may be right for you while using Tirzepatide.
• are breastfeeding or plan to breastfeed. It is not known if Tirzepatide passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby while using Tirzepatide.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Tirzepatide may affect the way some medicines work, and some medicines may affect the way Tirzepatide works.
Before using Tirzepatide, tell your healthcare provider if you are taking medicines to treat diabetes including insulin or sulfonylureas which could increase your risk of low blood sugar. Talk to your healthcare provider about low blood sugar levels and how to manage them.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How to use Tirzix Pre-filled Injection

Contraindications
•Personal or family history of medullary thyroid carcinoma or in patients with Multiple Endocrine Neoplasia syndrome type 2 (4)
•Known serious hypersensitivity to Tirzepatide(Tirzix) or any of the excipients in Tirzepatide(Tirzix).
Warnings and Precautions
•Severe Gastrointestinal Disease: Use has been associated with gastrointestinal adverse reactions, sometimes severe. Has not been studied in patients with severe gastrointestinal disease and is not recommended in these patients.
•Acute Kidney Injury: Monitor renal function in patients reporting adverse reactions that could lead to volume depletion.
•Acute Gallbladder Disease: Has been reported in clinical trials. If cholecystitis is suspected, gallbladder studies and clinical follow-up are indicated.
•Acute Pancreatitis: Has been reported in clinical trials. Discontinue promptly if pancreatitis is suspected. Do not restart if pancreatitis is confirmed.
•Hypersensitivity Reactions: Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported postmarketing with Tirzepatide(Tirzix). If suspected, advise patients to seek medical attention and discontinue Tirzepatide(Tirzix) promptly.
•Hypoglycemia: Concomitant use with an insulin secretagogue or insulin may increase the risk of hypoglycemia, including severe hypoglycemia. Reducing dose of insulin secretagogue or insulin may be necessary. Inform all patients of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.
•Diabetic Retinopathy Complications in Patients with Type 2 Diabetes Mellitus: Has not been studied in patients with non-proliferative diabetic retinopathy requiring acute therapy, proliferative diabetic retinopathy, or diabetic macular edema. Monitor patients with a history of diabetic retinopathy for progression.
•Suicidal Behavior and Ideation: Monitor for depression or suicidal thoughts. Discontinue Tirzepatide if symptoms develop.
Side Effects
The most common adverse reactions, reported in ≥5% of patients treated with Tirzepatide are:
